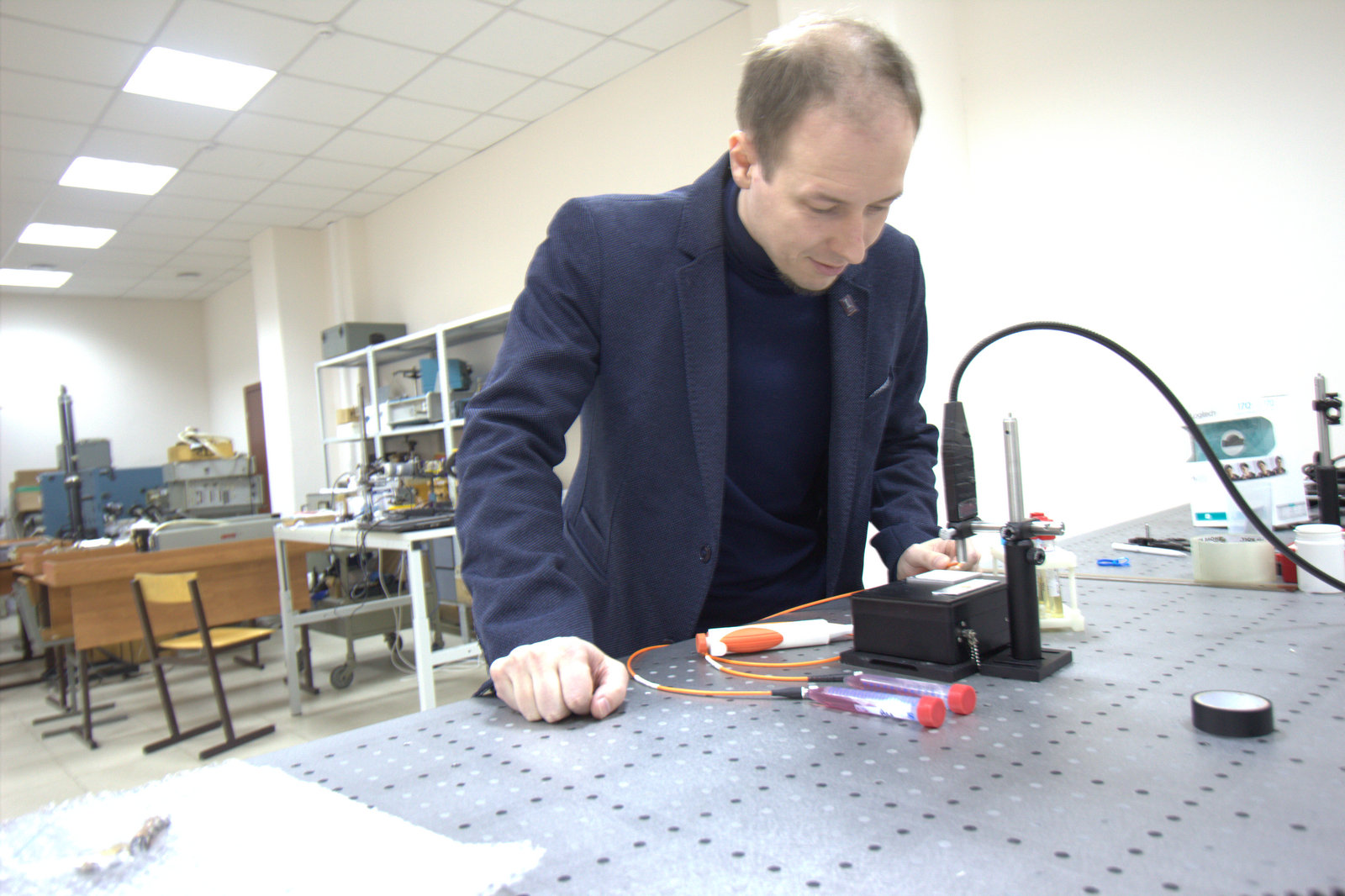
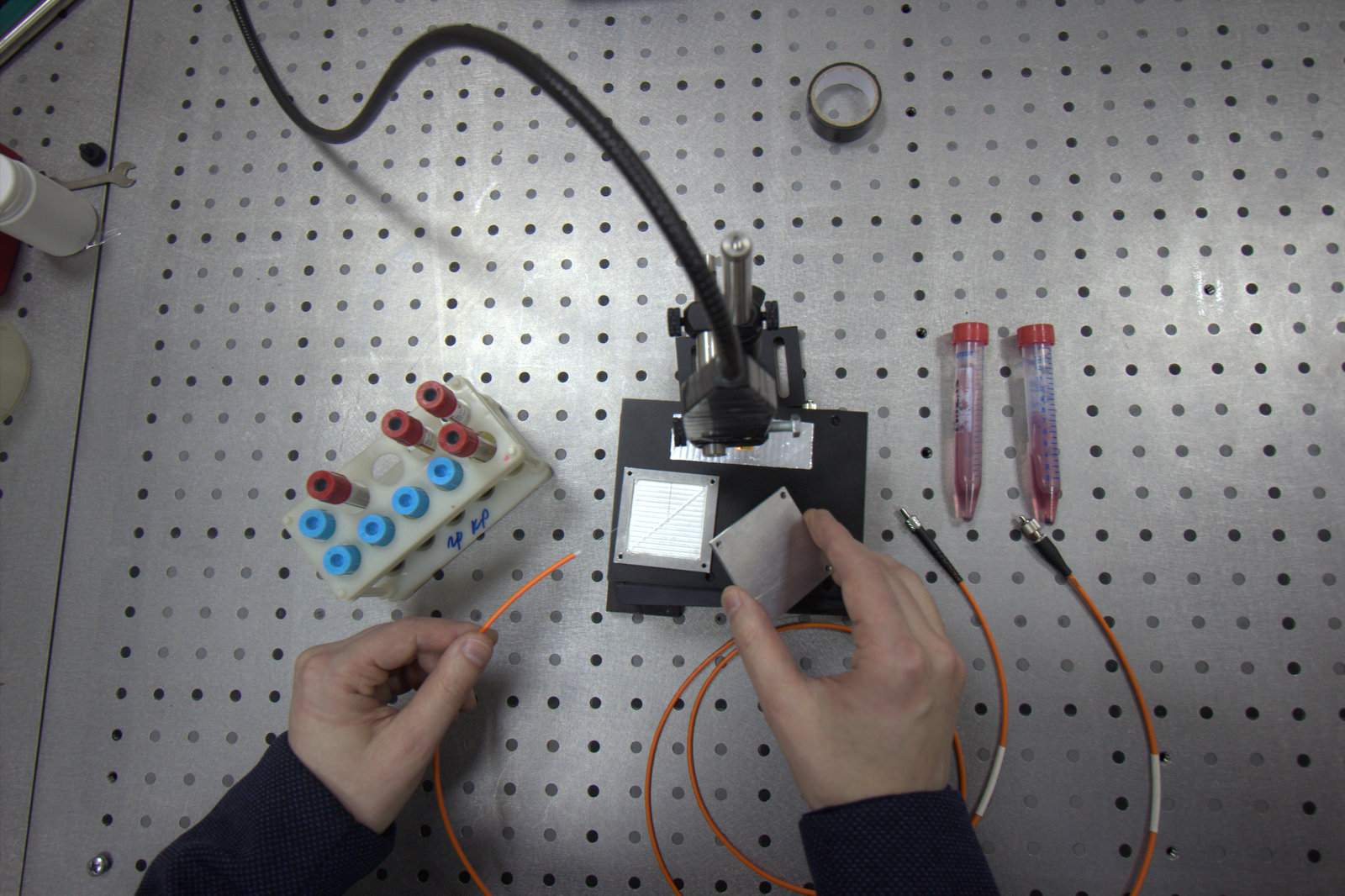
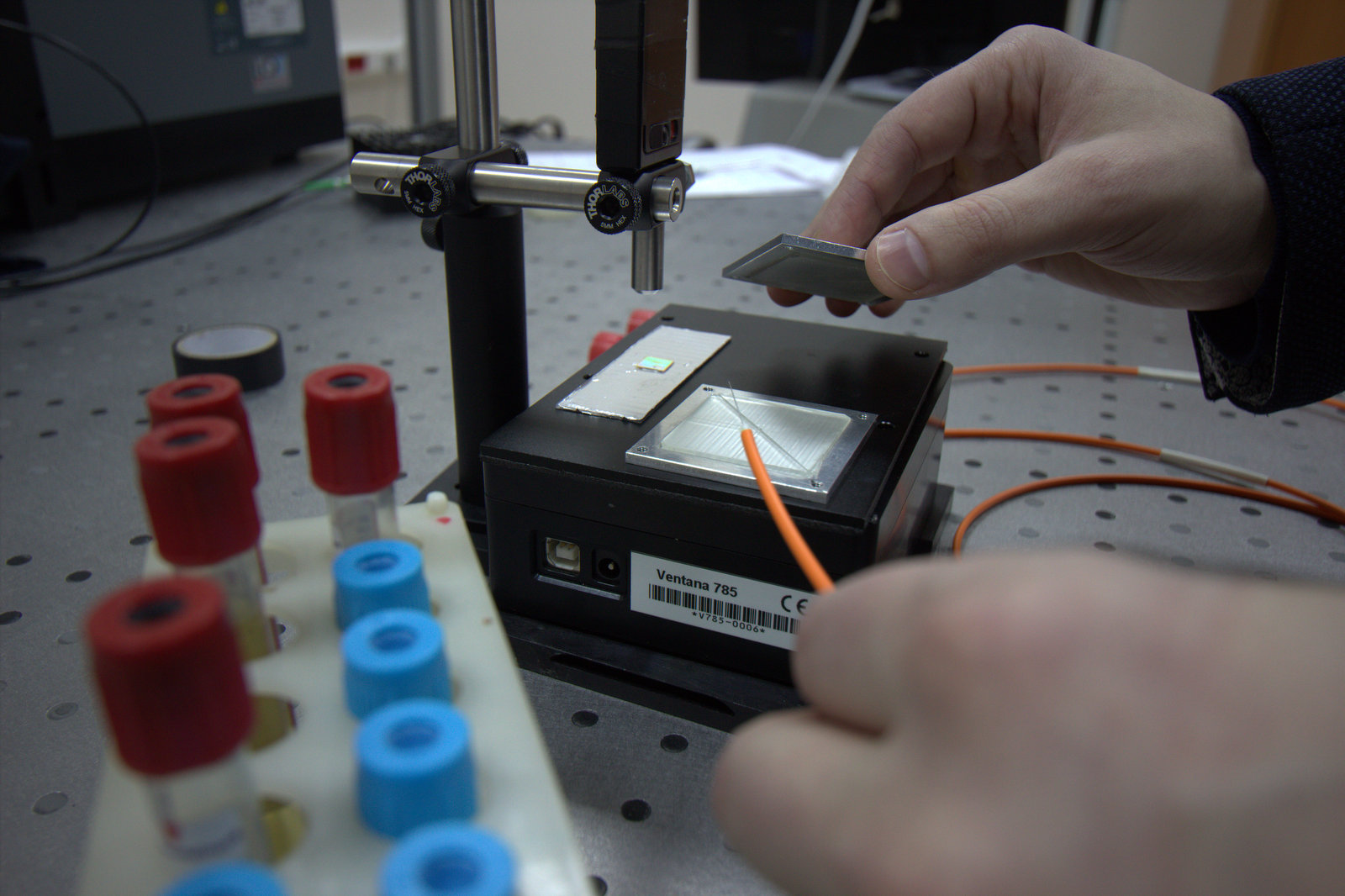
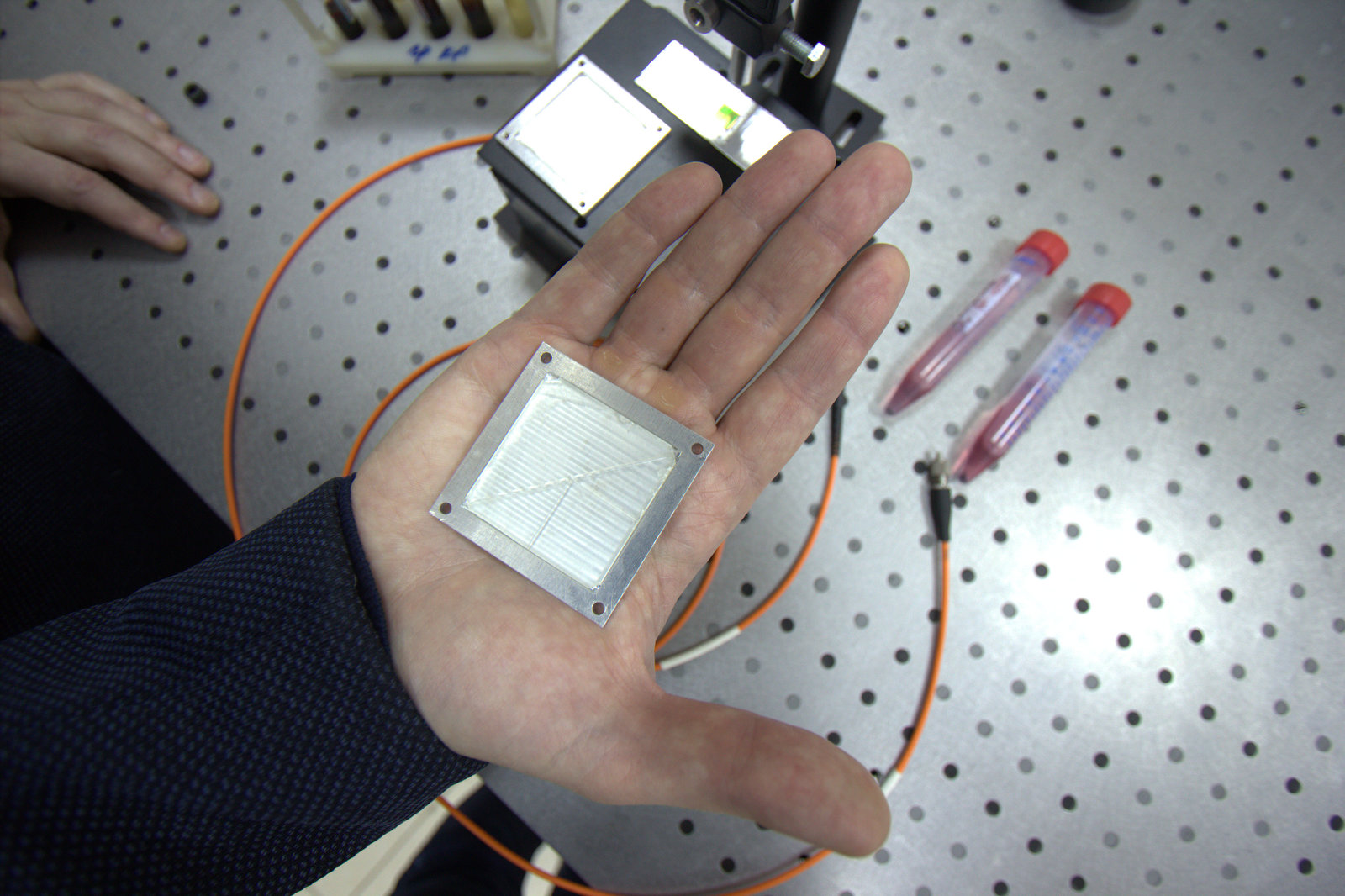
Doctors rarely express a categorical opinion. However, everyone agrees with the fact that early diagnosis is of great importance in the fight against oncology. Oncologists are in dire need of methods that are highly informative and accurate, and could be used for rapid screening.
Associate Professor of Сhemistry Department at Samara University Vladimir Platonov and Associate Professor of Laser and Biotechnical Systems Department at Samara University Ivan Bratchenko said why chemists teamed up with physicists to accomplish this task, and how the solutions found helped to create a micro-device for cancer detection.
By a drop
Tell us how the fusion of Physics and Chemistry can help in the fight against the disease of the 21st century - oncology?
V.P: The development of science has reached the level where it becomes obvious that to make a breakthrough you need to look for solutions at the junction of various disciplines. We went this way. And as a result of close cooperation of the Department of Chemistry and the Department of Laser and Biotechnical Systems, the technology providing fast and accurate non-invasive diagnosis of cancer has been created. This is a development of the total analytical system series, also known as "lab-on-chip". The lab on the chip is the flagship world trend in biochemistry, and our joint research is proceeding along this line. Our hybrid between microelectronics and a classical spectrophotometer is a microfluidic device, which will later be converted to a 20 by 20 mm chip. This is a complete analytical equipment in miniature, which will perform all operations with the sample, ranging from sample preparation to signal interpretation, as well as mathematical processing of the data that we will receive. To make it clear, earlier the similar equipment occupied huge laboratories.
You have mentioned that this is a non-invasive method, but how does your “laboratory” function?
I.B.: Yes, no intervention in the body is required, unlike a traditional biopsy. Now doctors are forced to take risks, removing a piece of tissue for research. In the later stages of the disease, this can cause extremely undesirable consequences, including metastasis, so one of the current trends is fluid biopsy.
What do doctors ideally want? To take a drop of saliva for research, analyze it and understand what is going on with a person. That is to get accurate information without the slightest harm to a person. It is clear that it is difficult to conduct such an analysis with one drop of saliva, but if we talk about blood, it is much more real. The fact is that tumour requires more nourishment than neighboring tissue; therefore, it often throws certain substances into the blood. It is impossible to catch them with classical optical methods. This is where the combination of methods of analytical chemistry and spectroscopy becomes useful. This is such a powerful tool that enables to detect even a single molecule, and understand what it is. Consequently, if at least one cancer cell appears in the body, which has begun its vital activity, it will inevitably release a certain marker into the blood, and this can be traced.
No magic, only chemistry
Is this really possible? Just from the first affected cell? How exactly do you track it?
V.P.: Yes, certainly, the task is difficult, but feasible. The effect is achieved through a combination of laser technology and analytical chemistry. Unlike purely optical methods, where the analysis is based only on a photograph, we perform chemical analysis using various spectral methods and determine the presence and concentration of certain substances in the blood, saliva and other biological fluids.
Microfluidics helps us in this. This science studies the patterns of behaviour of liquids moving through narrow channels inside sealed miniature devices - chips. At the Department of Chemistry, we are engaged in the creation of microchannels on the plane that is chemically modified and functionally tuned to the desired analytical task.
In addition, in order to make our lab-on-chip technology more informative, we are closely studying and developing various functional nanoparticles that are used in spectrophotometry. Our department has a whole scientific direction dedicated to this subject.
In its true light
How do nanoparticles affect the informativeness of the analysis?
I.B.: Let us consider the example. If we affect the blood with light, we will naturally see something, but the radiation will be very unfocused and implicit. However, if we adsorb functional substances that are in the blood plasma onto the surface of metal nanoparticles, then the radiation will become much more intense and the informative signal will be clearer with the same exposure to light. Basically, if we shine a laser onto a sample, the laser radiation will interact with the molecule in a certain way and the presence of metal nanoparticles will enhance this interaction by many times: tens, hundreds of thousands of times. This way we increase the accuracy and the minimum detectable concentration of toxic substances or some metabolites that indicate the state of the body. That is why the giant Raman scattering (the name of the approach) gives the result where conventional methods are powerless.
How do you manage to use giant Raman scattering when examining a micro dose of sample?
I.B.: Our laboratory is miniature and this imposes certain limitations on us: we have to work in a very small volume. We need to focus the radiation and for this purpose we will use the lens as an element of the chip. At Samara University there are modern technologies of chemical etching and CNC processing, and laser lithography, allowing to make all the necessary elements to detect giant Raman scattering.
Analytical chemistry and optical methods are mainly aimed at improving the accuracy of the analysis in your development, what is such duplication for?
V.P.: It is all about the goals you need to achieve. If we are talking, for example, about another development of the Chemistry Department - a gas microchromatograph intended for measurements in the field of ecology, here other concentrations are sufficient for high-precision analysis. If we compare them we can see that the concentration determined by a gas microchromatograph is 1 molecule per million others, maximum 1 molecule per 10 million, while the concentration for medical research is 1 molecule per trillion others, and this is not the limit. Therefore, such complex tricks in the form of a lens system, various chemical modifiers, as well as high-precision spectrometers with good wavelength resolution are necessary. That’s why we can "catch" undesirable "elements" in the body when they are just beginning their activity.
Bright future
What other advantages does your development have in addition to high accuracy?
V.P.: The device will be inexpensive. Cheaper prices are achieved due to miniature sizes: when processes occur in a 1 mm by 1 mm camera with a volume of less than one microliter much less expensive chemical reagents and trained laboratory technicians will be required, and the expenses on iron will not be needed here. In addition, reducing the size makes the device more accessible to a person. An ambulance doctor, for example, will be able to carry it even in his pocket, because the size of the future device will be approximately the same as that of a mobile phone, and in a couple of minutes he will not only conduct a complex laboratory study, but he will also instantly receive the result.
Does it mean that it will be possible to undergo rapid diagnosis of oncological diseases, for example, during the usual visit to a therapist?
V.P.: Yes, sure. Our development is intended primarily for professionals, it is invaluable in clinics, in the ambulance service. It is ideal for routine analyses, a great amount of which is being carried out among the population. This accurate, fast and cheap screening method is the method, which is so necessary for oncologists.
What stage is your research at now, what are its prospects?
I.B.: We have already created a laboratory setup for the research and are at the experimental stage now. Experimental studies, as well as, in fact, the development of the device itself are conducted in close tandem with the specialists of Samara State Medical University, as well as Samara Regional Clinical Oncology Dispensary. It should be noted that our joint development has a wider range of possibilities, and is not limited to the tracking of biomarkers that are responsible for oncology. It is possible to measure glucose levels, and determine the presence or absence of toxic substances in the blood, etc. Based on the proposed technology, we will be able to create a whole series of devices that will be cheaper, better, more accurate and much more compact than all existing ones.
 RU
RU  EN
EN  CN
CN  ES
ES